Dr. Sean Roberts, a faculty affiliate of the Texas Materials Institute, and his group have had their work on lead halide perovskite quantum dots recently published in ACS Energy Letters. The team studied how different variants of naphthalene energy acceptors stick to these dots, which impact their ability to facilitate photon upconversion, a process wherein low-energy visible photons are used to drive emission of high-energy UV light. The production of UV light via this process has several potential uses, such as initiating chemical reactions and facilitating high-resolution 3D printing.
Working together with synthetic collaborators in the Page group at UT Austin, the Roberts group found that some acceptors, such as those that use amine binding groups, do not stick well and therefore do not facilitate photon upconversion. However, they found that others - especially 1-naphthoic acid - stick better than other acceptors, which improves their ability to facilitate the generation of UV light.
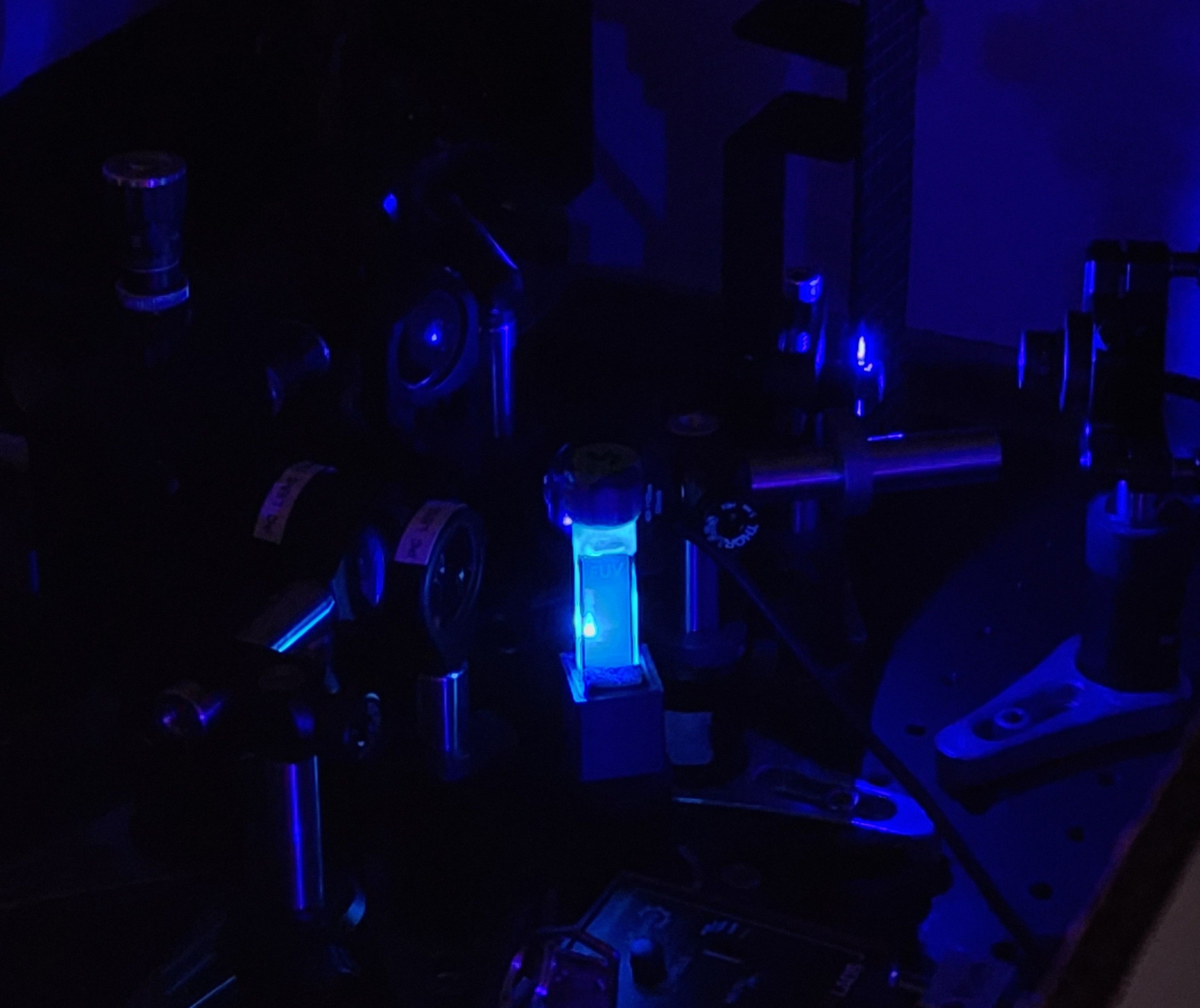
The upconversion is from blue to UV light, making it difficult to pick up by the eyes or camera lens. Above photo by Xinyi Wu, a graduate student in the Page group and lead author of the article.
The key takeaway: how well these molecular energy acceptors attach and interact with quantum dots makes a big difference in how well photon upconversion works. This insight can help scientists design better materials for advanced technologies.
Read more of their article, "Through-Bond Electronic Coupling and Strong Ligand Binding Facilitate Blue-to-UV Photon Upconversion by CsPbBr3 Quantum Dots," at ACS Energy Letters.

