A newly published article in Advanced Materials was co-authored by several researchers affiliated with the Texas Materials Institute, including Yixian Wang, Vikalp Raj, Rohit Raj, Hugo Celio, Andrei Dolocan, and TMI core faculty member David Mitlin, along with collaborators from across the field.
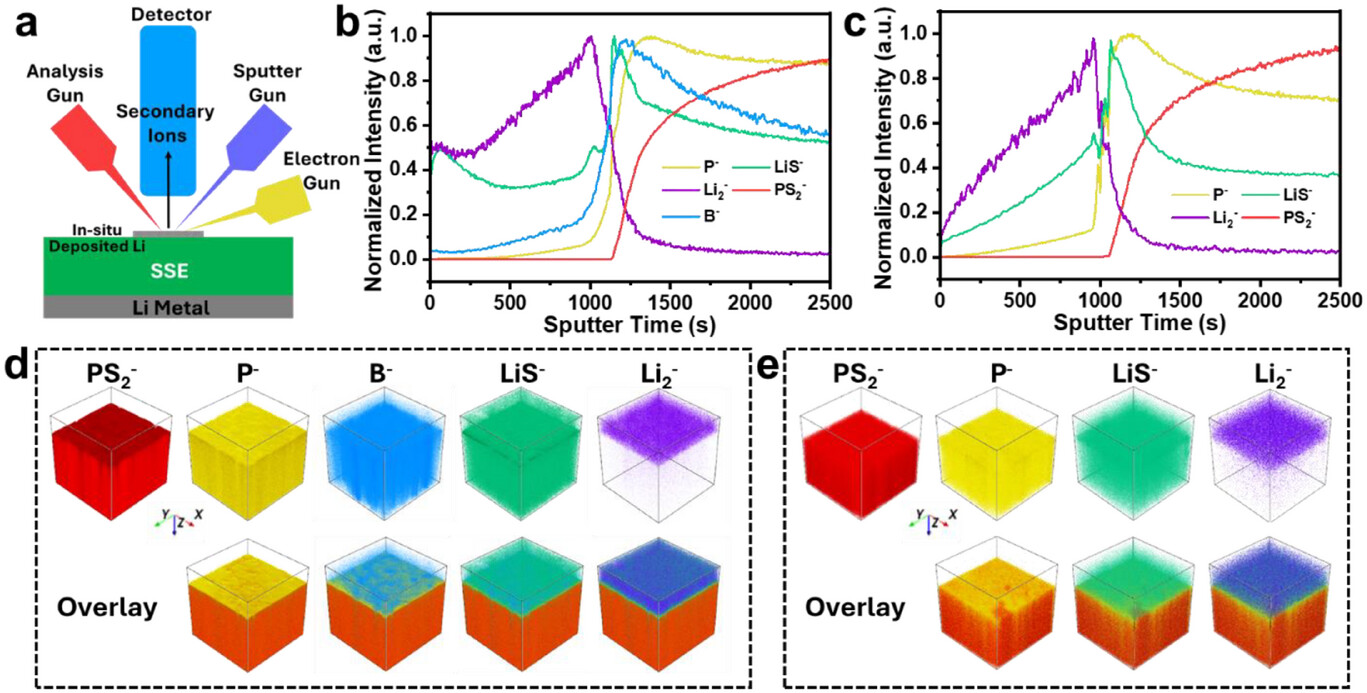
"Understanding the Role of Borohydride Doping in Electrochemical Stability of Argyrodite Li₆PS₅Cl Solid-State Electrolyte" investigates how borohydride doping impacts the electrochemical stability of argyrodite-type solid-state electrolyte, a key development in the pursuit of safer and more efficient solid-state batteries. The researchers found that adding lithium borohydride to the electrolyte improves the battery's performance, lifespan, and efficiency—especially under high current, long-term use, and cold conditions—without requiring major changes to the electrolyte’s basic structure. The key to this improvement lies in the way the new SEI layer forms and protects the battery’s surface.
The connection between lithium metal and the electrolyte (Li6PS5Cl, or LPSCl) is very important for the stability of solid-state batteries (SSBs) because this connection tends to be unstable. This study shows that adding lithium borohydride (LiBH4) to LPSCl improves its stability and overall performance. To understand why this happens, the study used advanced techniques to look at how lithium deposits onto the electrolyte’s surface. These tests showed that the borohydride doping helps create a better protective layer (the SEI), which stabilizes the surface of the battery.Theoretical modeling shows that the borohydride doesn’t change the basic stability of the electrolyte too much—it just helps the battery work better by improving the way lithium interacts with the electrolyte.
Read more in their article, which can be found at Advanced Materials.